
Digoxin Toxicity
Clinical Assessment Tool

Select suspected clinical condition of the patient.
ACUTE
Digoxin Toxicity
CHRONIC
Digoxin Toxicity
Has there been a known ingestion of digoxin (suicidal or accidental) of ≥10 mg in a healthy adult or >4 mg in a healthy child?*
*Suicidal ingestion may involve more than one drug. Toxic effects of other drugs or poisons should not be overlooked.
Is there clinical suspicion for digoxin toxicity?

Select all that apply.
Current or recent digoxin use and at least one of the following:
Other clinical suspicion for digoxin toxicity
If digoxin toxicity is not suspected…
Review the patient’s history, treat their symptoms, and explore other causes.
Are there signs or symptoms of potentially
life-threatening digoxin toxicity?
Are there signs or symptoms of potentially life-threatening digoxin toxicity?
Are there signs or symptoms of potentially
life-threatening digoxin toxicity?
Are there signs or symptoms of potentially life-threatening digoxin toxicity?

Select all that apply.
Laboratory parameters
*
Samples should be collected at least 6-8 hours after the last dose of digoxin to allow for equilibration between serum and tissue.
Are there signs or symptoms of potentially
life-threatening digoxin toxicity?
Are there signs or symptoms of potentially life-threatening digoxin toxicity?
Select all that apply.
Evidence of end-organ dysfunction from hypoperfusion
If no signs or symptoms of digoxin toxicity are observed…
Monitor the patient, repeat laboratory tests, and perform serial examinations.
Any sign or symptom of potentially life-threatening
digoxin toxicity may indicate the need for immediate intervention with DIGIFab.
Any sign or symptom of potentially life-threatening digoxin toxicity may indicate the need for immediate intervention with DIGIFab.
To determine dosing for patients with acute digoxin toxicity, identify if the amount of ingested digoxin is known or unknown.
Any sign or symptom of potentially life-threatening
digoxin toxicity may indicate the need for immediate intervention with DIGIFab.
Any sign or symptom of potentially life-threatening digoxin toxicity may indicate the need for immediate intervention with DIGIFab.
To determine dosing for patients with chronic digoxin toxicity, identify if a steady-state serum digoxin concentration is known or unknown.
For patients with acute digoxin toxicity and a known amount of ingested digoxin, the dose of DIGIFab can be calculated based on total digoxin body load.
Total digoxin body load* (mg)
x
0.8
0.5 mg
*Total body load for digoxin tablets is approximately equal to the amount ingested (in mg) x 0.8 (bioavailability of the tablet preparation).
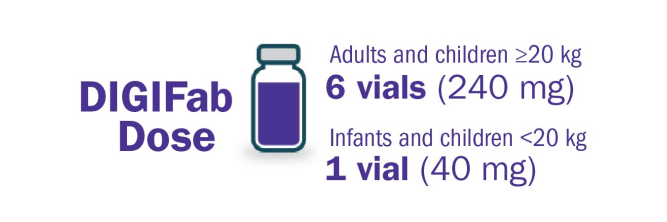
Dosing for patients with acute digoxin toxicity and an unknown amount of ingested digoxin:


- Monitor for volume overload in small (<20 kg) children
- Initiate with 10 vials followed by an additional 10 vials, if needed, to avoid a febrile reaction
For patients with chronic digoxin toxicity and a known steady-state
serum digoxin concentration, the dose of DIGIFab can be calculated based on serum digoxin concentration* and patient weight.
Serum digoxin (ng/mL)
x
weight (kg)
100
*Samples should be collected at least 6-8 hours after the last dose of digoxin to allow for equilibration between serum and tissue.
Dosing for patients with chronic digoxin toxicity and an unknown steady-state serum digoxin concentration:

Results
The patient characteristics you selected are consistent with potentially life-threatening digoxin toxicity. Early recognition of digoxin toxicity is essential and may result in improved treatment outcomes.

For potentially life-threatening digoxin toxicity, think R.A.P.I.D.
Recognize. Act. Promptly Infuse DIGIFab.

BTG and the BTG roundel logo are registered trademarks of BTG International Ltd. DIGIFab is a registered trademark of BTG International Inc. 2021 BTG International Inc. All rights reserved. US-DGF-2100056 October 2021

 Access Dose Calculator
Access Dose Calculator